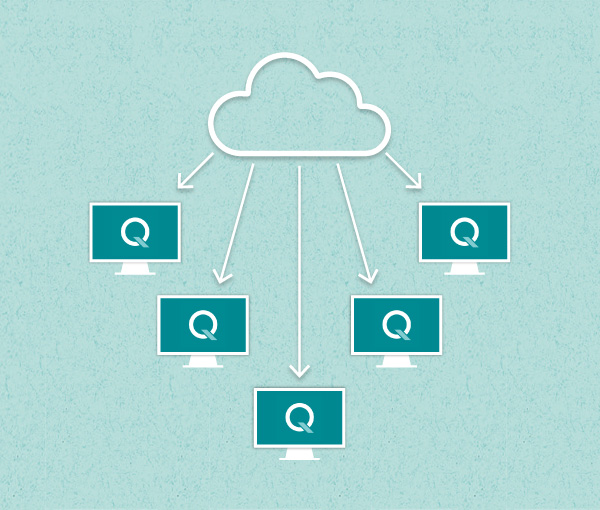
q-doc® – Data Management Software
Single workstations and networked solutions with built-in data integrity.
- 100% scalable 3-layer software architecture with central MS-SQL database
- Powerful framework with device-independent methods that can be used for different instrument types
- Modules extend the basic functionalities with LDAP integration, LIMS export, data trending, and more
- More than 35 drivers for dissolution and physical testing instruments
- Report, check & evaluate data directly in q-doc and sign with electronic signatures
- Easily create complete batch reports and compare batches over time intervals
- Human readable audit trail and product version control for full traceability and compliance
- Fulfills all requirements for implementation of a 21 CFR part 11 compliant system
Highlights

Your Data. Simply Managed.
Actively managing your data saves costs and ensures regulatory compliance. Avoid error-prone manual transcription of test results and other time-consuming activities to consolidate test protocols. Depending on your data management requirements, test systems can be operated stand-alone or networked, allowing you to flexibly choose your desired level of integration.

All your data in one place.
Take full control of your data and manage all your methods, results, and users with q-doc®. Use the same method on different systems, consolidate data from multiple test runs in a single report, and avoid redundant management of users and their passwords. Whether operated with a single PC workstation or in a networked environment, the modular q-doc® data management software offers built-in data integrity and ease of regulatory compliance. From electronic signatures, audit trail, and advanced user management to LDAP integration, batch comparison, and LIMS import / export functions – the q-doc® framework is designed for implementation of an efficient, fully 21 CFR part 11 compliant system.
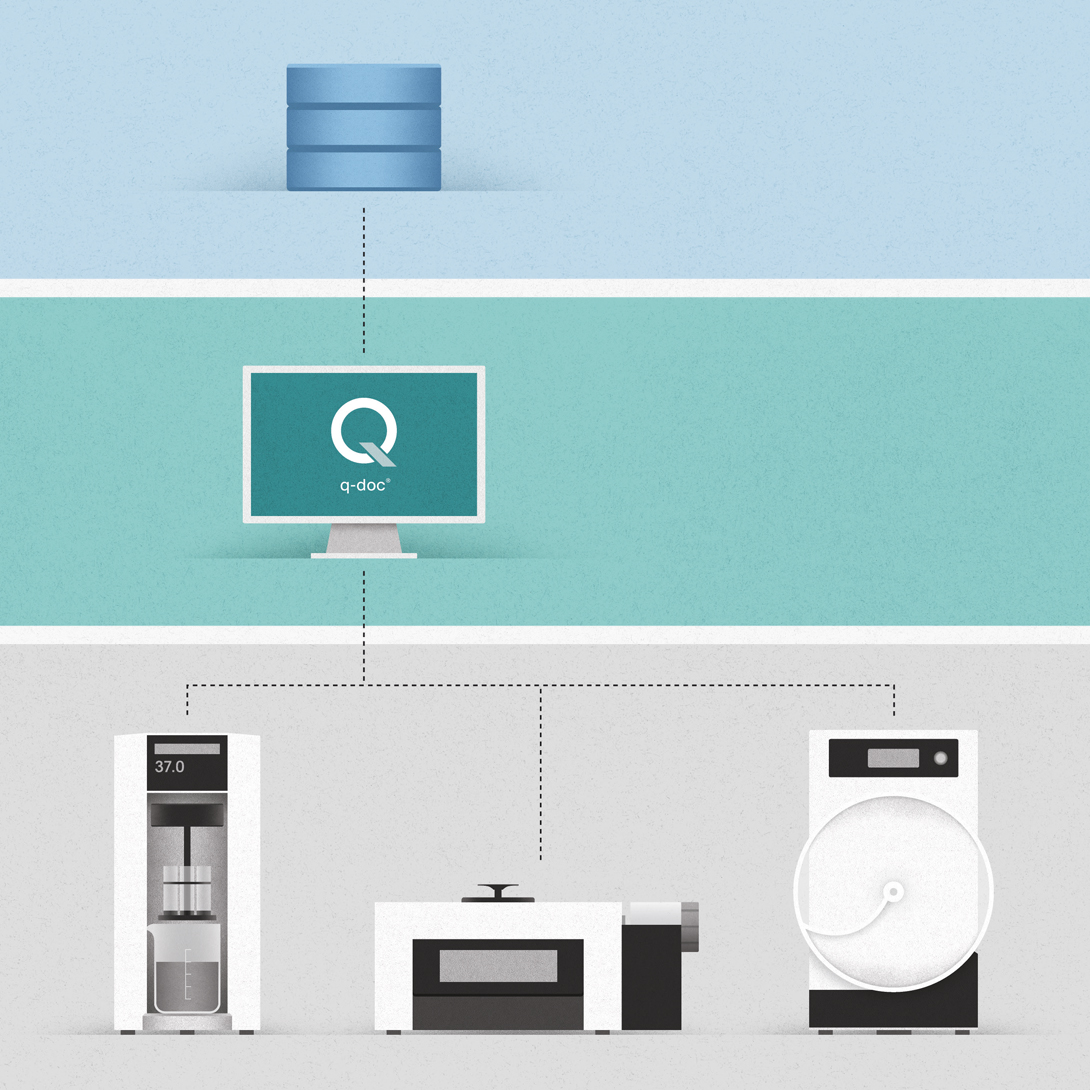
Framework – All key functions.
Whether installed on a single PC or on multiple networked computers, the q-doc® framework with central SQL database is designed for both stand-alone and networked operation alike. It includes all basic functions to collect, record, analyze, evaluate, report, and manage test data in full compliance with 21 CFR part 11 requirements. To simplify daily processes, login with chip ID, barcode printing, and touchscreen-supported operation are standard.

Modules – Extend as needed.
Modules further extend the q-doc® framework with import / export functions, LDAP integration for simplified user administration by IT administrators, data trending and batch comparison to analyze results over a period of time, data evaluation including re-test procedures according to predefined USP and Ph. Eur. specifications, and other data management possibilities to simplify operation in large interdisciplinary networks.
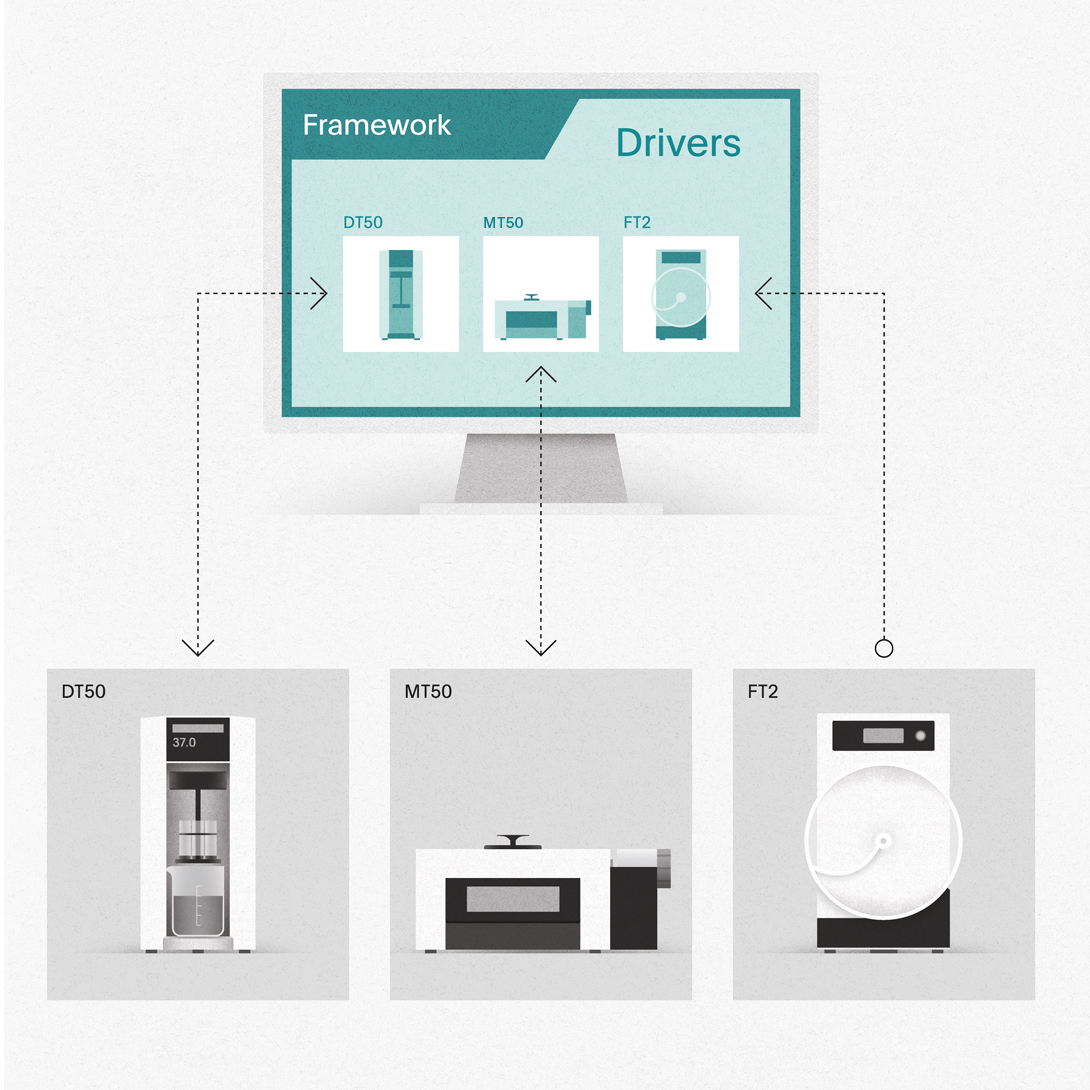
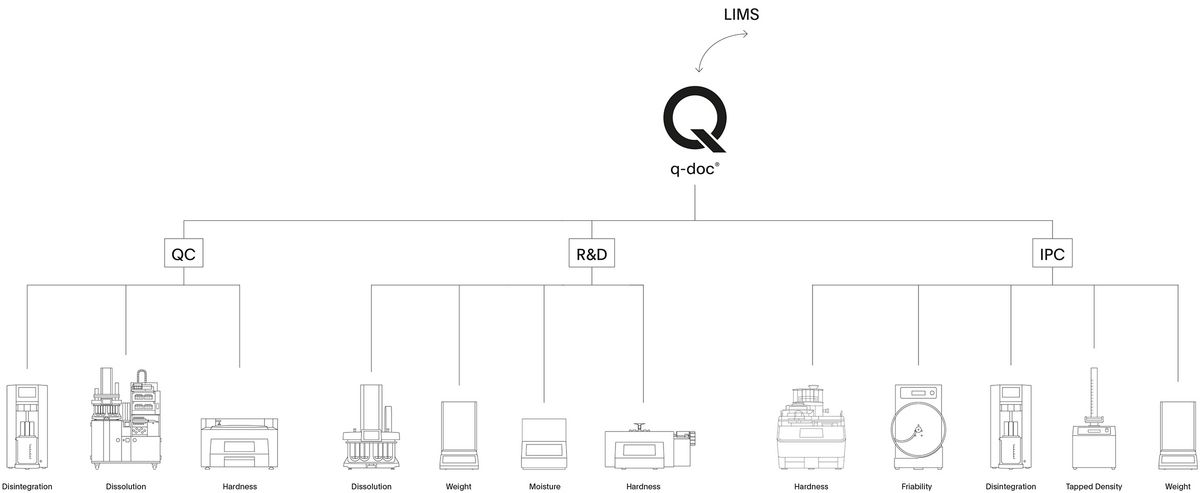
Drivers – Connect your instruments.
Record weight measurements from your balances, connect different physical and dissolution testing systems, and seamlessly protocol everything in one place. Standard q-doc® drivers for more than 35 different instrument types and system configurations are readily available for establishing a highly efficient data management system in R&D, QC, and IPC departments.

Process Drivers – Complete your electronic records.
While an ‘instrument driver’ allows to perform tests with a specific type of instrument, a ‘process driver’ primarily ensures that specific steps for a certain type of test are followed. The operator is guided through the testing process and all steps are documented automatically. This is particularly useful for procedures that require executing a lot of manual tasks.

Future-Proof Your Data Management with q-doc®
Ensure seamless operations with q-doc® Software Support Contracts. Benefit from free updates to stay ahead of tomorrow’s challenges and priority service for fast, hassle-free support when you need it most. Avoid costly delays and inefficiencies—upgrade confidently and keep your processes optimized.

Seamlessly connect IPC with your MES.
The new standard interface between PAS‑X MES and q‑doc® allows results from multiple IPC tests performed at different manufacturing steps to be captured, managed, and automatically sent to the MES in one central location. This brings test results, ranging from raw material powder characterization to final dosage form testing, directly into a production context. This simplifies processes and increases compliance.
Modules
Job Import
LIMS Export
LDAP Integration

Data Trending
Workgroups

Extended Evaluation